Sistema de monitoreo de datos inalámbrico
Nuestro sistema de monitoreo de datos inalámbrico
monitors temperature and humidity in the pharmaceutical sector. Compliant with GxP and CFR Part 11
Comprehensive monitoring of environmental parameters for regulatory compliance and operational excellence.
Audits, checks, norms: There are few commercial areas which are as strictly regulated and monitored as the pharmaceutical sector.
As a global leader in sensor techology, Testo has almost 70 years of measurement engineering experience. Testo supports hundreds of thousands of data loggers around the globe with over 17 billion data sets available to clients daily.
Our wireless data monitoring system:
- Integrated system comprising sensors, software and services
- Seamless recording and documentation of all environmental parameters, including: temperature, humidity, and differential pressure
- Ensure Compliace with GxP and 21 CFR Part 11 requirements
With ou wireless data monitoring system, you have the certainty that your measurement data are recorded securely and regulatory requirements reliably fulfilled. With the confidence you gain in knowing your Pharmaceuticals are seamlessly monitored, you can concentrate on the more profitable tasks.
Functions and advantages
Testo offers all the instruments required for continuous environmental monitoring
 The function
The function
Automated and uninterrupted measurement and documentation of:
- Temperature
- Humidity
- Differential pressure
Additional sensors (e.g. door open) can be flexibly integrated into the system.
Your advantages
- Reliable monitoring of relevant parameters
- More flexibility thanks to a system that can expand over time to meet any scale
More security: Fully automatic and with minimum effort
 The function
The function
- Complete automatic recording, transmission and storage of the measurement values
- Alarms and documentation according to pre-definition, with no manual effort
Your advantages
- More security thanks to automation and ability to react in real time
- Highest possible level of reliability thanks to a minimum of manually necessary actions
- Savings of costs and time thanks to low resource involvement
Advance alarm setting: In real time and via various channels
 The function
The function
- Real-time alarms in case of limit value violations and critical system events
- Operator note section for tracking corrective actions
- Set by shift and create hierarchy escalation based on custom criteria
- Optical warning signals on data loggers and Base station
- By SMS, e-mail and as a notification on smartphone, tablet or PC
Your advantages
- Alarms and reaction in real time: Intervention before damage occurs
- Alarms sent to people who can take action based on shift time and skill
- Location-independent alarms and access at all times and from anywhere
Scalability: Building, campus, region, country, or global expansion and oversight
 The function
The function
- Unlimited scalability: With just one system, you can effortlessly monitor multiple sites across campus, or around the world
- Web-based cockpit for mobile access to the measurement data and system alarms
Your advantages
- Best possible transparency over GxP relevant environmental parameters
- Scalability from local to global in just one system, for more flexibility
- Location-independent access to improve efficiency.
Data integrity: You can count on that
 The functions
The functions
- Redundant data storage: Logger, Base & database / software
- Strong user management with authentication to manage user rights
- Audit trail
Choose between two software packages:
- PRO Software: Ideal for automated and seamless environmental monitoring of cross-location areas which are subject to less strict regulations
- GxP Software: Validatable according to the GAMP5 regulations and compliant with the FDA’s 21 CFR Part 11 requirements and GxP guidelines.
Your advantages
- Redundant data storage ensures it is always there for you
- Compliance & conformity with FDA 21 CFR Part 11 and GxP guidelines
- Validatable software (according to GAMP5)
- Uninterrupted and tamper-proof documentation
Sensors, software, services: All from one vendor
 The functions
The functions
- Excellent fusion of hardware, software and services
- Testo is your partner: In installation, commissioning, calibration and IQ/OQ/PQ documentation
Your advantages
- One provider for all project-related topics
- Extensive service offer:
- Project management
- Installation and commissioning
- Site Acceptance Test
- Thermal and periodic mapping
- Calibration and recalibration
- Software updates as well as hardware backups
- Training
- Hotline and exchange instrument service
Together, we define your individual testo Saveris Pharma service package.
Modular and flexible: Ready for every application
 The functions
The functions
- Like modular building blocks, testo Saveris Pharma adapts perfectly to your conditions
- Communication standards supported: WLAN, LAN and testo UltraRange (radio)
- Connections for various digital and analog probes
- Digital probes with a minimum servicing effort
- Robust, strong radio signal with extraordinary range
Your advantages
- Whatever you need to do: The system adapts and grows with the task
- Probe calibration during continuing operation without loss of data
- Secure coverage of all applications and measuring ranges
Backwards compatible: Innovation meets proven technology
 The functions
The functions
- Compatibility of the new Base station of the 3rd generation testo Saveris Pharma with the previous generation of the testo Saveris Pharma system is no problem
- A replacement of the entire system is not necessary in order to use the new Base
- Further replacement of new hardware components step-by-step
Your advantages
- Safe decision: Service and updates beyond 2026
- More efficient measurement: Integration of the new modular testo Saveris Pharma data loggers
- Improved scalability. Smooth integration of up to 1000 data loggers and 3000 measurement channels
- More security: SMS alarms via LTE stick
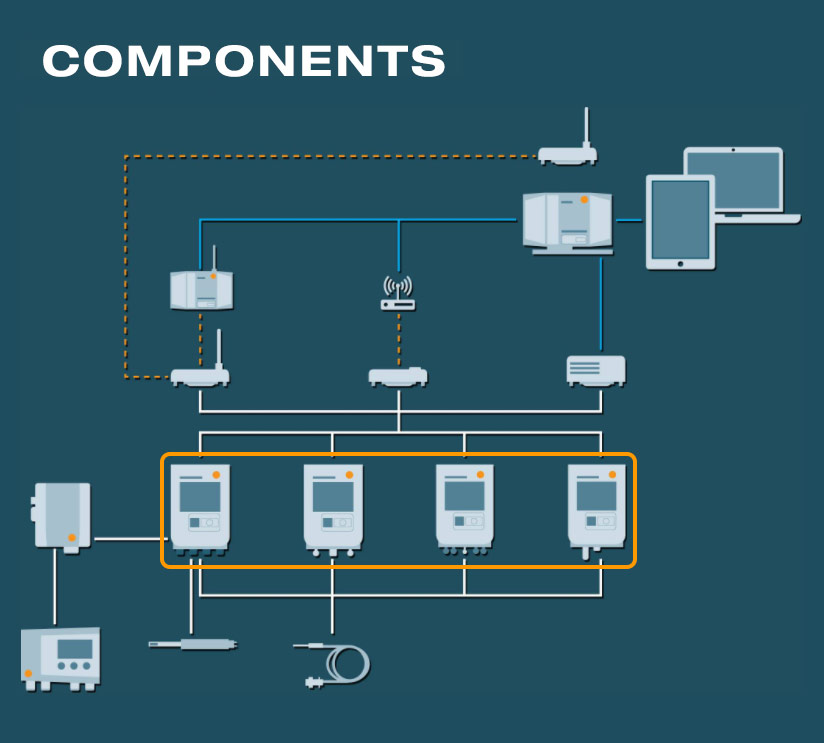
The components
The data logger modules
Every data logger can be flexibly combined with the three communication modules (WLAN, LAN, testo UltraRange)
- testo 150 TUC4: Four connections for digital probes via Testo Universal Connector
- testo 150 TC4: Four connections for thermocouples for applications in extreme conditions
- testo 150 DIN2: Two connections for standard probes via miniDIN for using the comprehensive Testo probe range
- testo 150 T1: Internal NTC temperature sensor for monitoring temperature

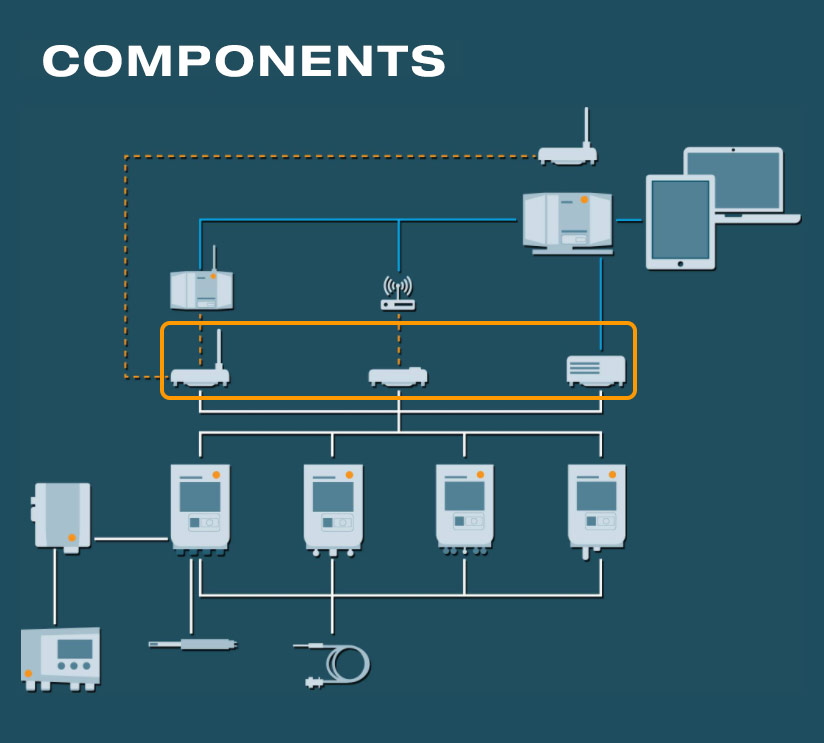
The communication modules
The communication modules allow the use of different communication technologies with the testo 150 data logger modules:
- Existing infrastructure (WLAN or LAN/Ethernet)
- testo UltraRange long-range radio technology
Even after commissioning, communication and data logger modules can be flexibly connected with each other and exchanged.

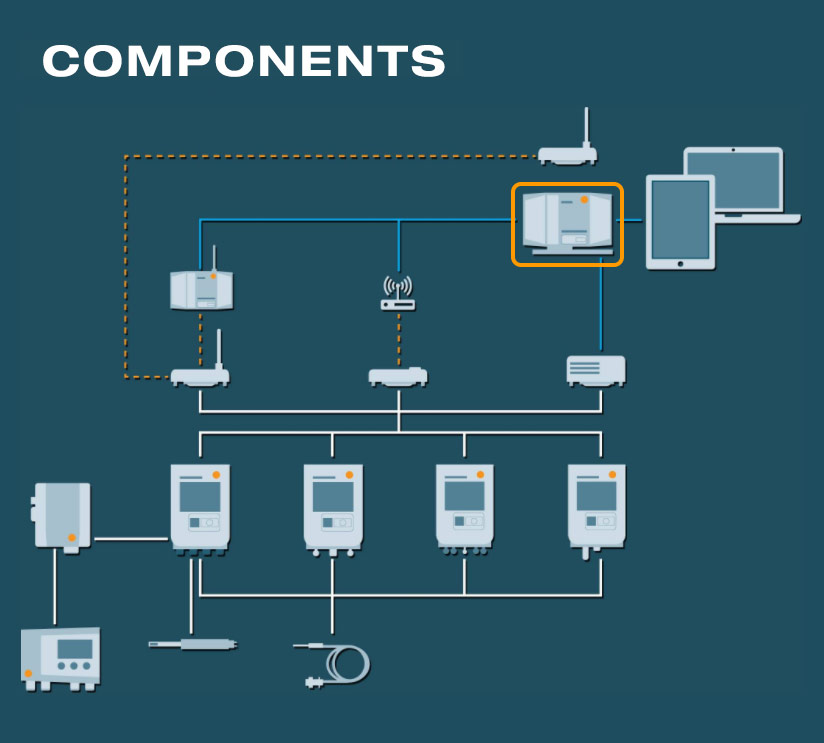
The Base station
- The heart of the testo Saveris Pharma environmental monitoring system
- testo UltraRange functionality via additional communication module
- Management and analysis of the measurement values of up to 3,000 channels
- GxP-compliant alarms in cases of limit value violations or critical system events via integrated LED or connected alarm provider (via relay)
- Integrated emergency battery for highest data security in case of power cut or other system malfunctions

The Gateway and the testo UltraRange technology
The gateway
Access point and transmission support for communication via the independent radio network testo UltraRange with encrypted, proprietary signals.
testo UltraRange radio technology
- Developed for use in buildings
- Excellent range and signal robustness
- Depending on the customer’s wishes, the connected components are wirelessly updated

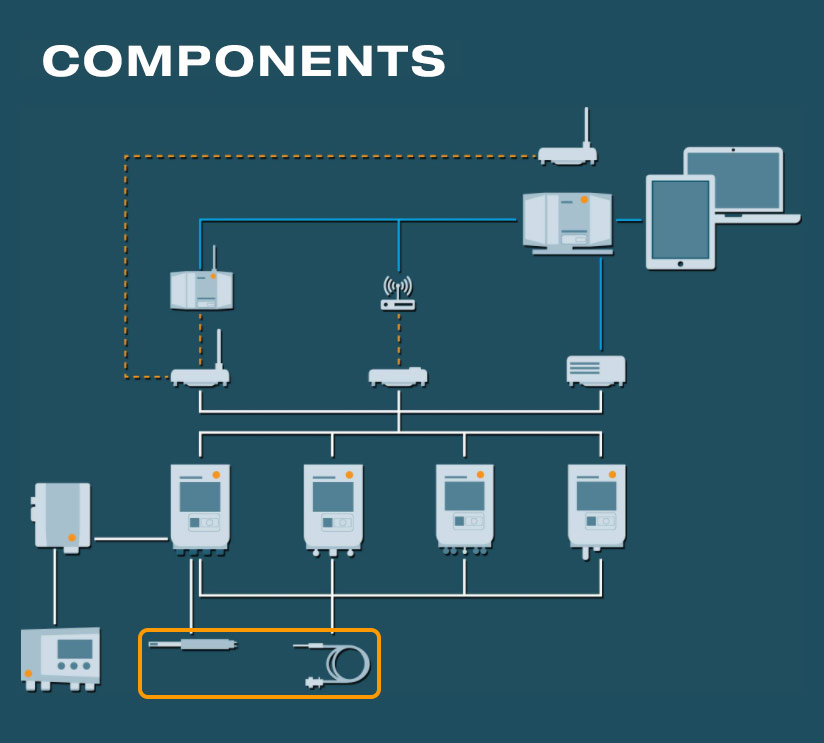
The digital and analog probes
Digital and analog temperature and humidity probes for high-precision measurements of GxP-relevant parameters in a regulated environment.
- Measuring range from -200 to +1300 °C for almost any possible scenario in the pharmaceutical sector
- Probe exchange in seconds without data gaps in the documentation
- Easy handling and installation
- Efficient system monitoring with digital door contacts

Transmitter for humidity and differential pressure
- Integration of additional measurement parameters like humidity or differential pressure
- Ideal solution for highest accuracy and for special applications (high humidity, trace humidity, etc.) in compressed air, drying and air conditioning technology as well as in cleanroom applications

Software and cockpit
The testo Saveris Pharma software is available in two different versions:
- PRO Software: For automated and seamless environmental monitoring of cross-location areas which are subject to less strict regulations
- GxP Software: Validatable version with Audit Trail, electronic signatures and user levels with differing user rights (conformity with 21 CFR Part 11)
The web-based cockpit:
- Web-based and intuitive user interface
- Identify and acknowledge alarms at any time, location-independently and from any end device (smartphone, tablet, PC)
- Platform-independent data access
- Integration of individual floor plans for easy and fast localization of measurement locations and alarms

The applications areas
Smooth processes and convenient documentation
 testo Saveris Pharma is used in medical, bio-technical, chemical and pharmaceutical laboratories and cleanrooms. Our customers depend on the system to monitor important environmental parameters, maintain high quality standards and ensure traceability.
testo Saveris Pharma is used in medical, bio-technical, chemical and pharmaceutical laboratories and cleanrooms. Our customers depend on the system to monitor important environmental parameters, maintain high quality standards and ensure traceability.
In Labs features:
- Reliable, automatic and continuous checks and monitoring of temperature, humidity and differential pressure
- Support in the adherence to different internationally valid quality standards such as Good Laboratory Practice (GLP) or DIN EN ISO 17025
testo Saveris Pharma has been used for years in these environmental conditions and rooms:
- (Research) laboratories
- Cleanrooms
- Facilities for animals
- Greenhouses
- Stability test chambers
- Bio-banks
- Blood and tissue banks
The system also monitors the temperature and humidity of appliances:
- Refrigerators, deep-freezers, ultra-deep-freezers, liquid nitrogen applications
- Other laboratory equipment such as water baths
Safe production of sensitive pharmaceuticals
 One of the most important applications of testo Saveris Pharma is the monitoring and documentation of the environmental conditions in the production of drugs, medication, APIs, bio-pharmaceuticals, tissue samples or medicinal products.
One of the most important applications of testo Saveris Pharma is the monitoring and documentation of the environmental conditions in the production of drugs, medication, APIs, bio-pharmaceuticals, tissue samples or medicinal products.
In production:
- Central and uninterrupted documentation of measurement data
- Comprehensive alarm management for fast reaction in cases of limit value violation or other critical system deviations
- A complete solution comprising sensors, software and comprehensive GxP services for cleanrooms, production, aseptic filling, packaging, intermediate and final storage of APIs, ancillary substances and finished products
The validatable environmental monitoring system corresponds to the ERES principle (Electronic Records, Electronic Signatures), and is thus compliant with the requirements of 21 CFR Part 11 for automated systems.
More security in storage and transport
 Well-known customers worldwide depend on testo Saveris Pharma not only in production – the system is also found in attached logistics centers, in order to adhere to international standards.
Well-known customers worldwide depend on testo Saveris Pharma not only in production – the system is also found in attached logistics centers, in order to adhere to international standards.
Our customers are thrilled that Testo not only provides the system for logistics applications, but also fully supports them in calibration, mapping, qualification and validation in the following areas of application:
- Warehouses and distribution centres
- Incoming goods
- High-bay warehouse
- Refrigerated rooms
- Refrigerators, deep-freezers, ultra-deep-freezers, liquid nitrogen applications
Certainty for hospitals and pharmacies
 testo Saveris Pharma is used in the health sector to protect the safety of patients and reduce the risk of product loss and compliance violations, through uninterrupted monitoring and documentation.
testo Saveris Pharma is used in the health sector to protect the safety of patients and reduce the risk of product loss and compliance violations, through uninterrupted monitoring and documentation.
- In the operating and treatment rooms of a hospital
- For protecting the samples in blood and tissue banks
- For norm-compliant environmental conditions in cleanrooms
- In in-house dispensaries where sensitive medication is produced and stored
testo Saveris Pharma is also used in the health sector for the monitoring of temperature and humidity in appliances:
- Refrigerators, deep-freezers, ultra-deep-freezers, liquid nitrogen applications
- Incubators
Validation Services: Ensuring Quality Compliance within Facilities
 Validation of critical equipment within life science facilities is essential to satisfy certain user requirements but also strict government regulations.
Validation of critical equipment within life science facilities is essential to satisfy certain user requirements but also strict government regulations.
It offers a wide variety of validation mapping services and products to help perform mappings on:
- Facilities
- Controlled Temperature Units (CTU)
- Clean Rooms
- Autoclaves
- Lyophilization
Testo can offer support in qualification projects and will work to develop a plan to reach the ultimate goal of complete regulatory and quality compliance.
Certainty, security, precision: Your advantages with testo Saveris Pharma
Wireless Data Monitoring System
![]()
Complete solution comprising sensors, software and services:
An experienced company. A reliable service partner. Proven technology.
![]()
Seamless recording and documentation of all relevant environmental parameters:
You can rely on the uncompromising functionality of the system.
![]()
Compliant with GxP and 21 CFR Part 11:
No buts – testo Saveris Pharma works strictly according to the most important requirements.
![]()
Alarms in real time:
Whatever happens – the system informs you immediately so you can intervene in time.